- Categories
- Tags
ISASS Policy Statement 2022– Systematic review of Intraosseous Basivertebral Nerve Ablation
This policy supplements the ISASS Guideline Statement – Intraosseous Ablation of the Basivertebral Nerve for the Relief of Chronic Low Back Pain (2020)
Morgan Lorio, MD, FACS,1 Olivier Clerk-Lamalice, MD,2 Milaris Rivera, MD,3 Kai-Uwe Lewandrowski, MD,4
1 Advanced Orthopedics, Altamonte Springs, Florida
2 Beam Interventional & Diagnostic Imaging, Calgary, Canada
3 Universidad Autónoma de Guadalajara, School of Medicine, Zapopan, Jalisco
4 Center for Advanced Spine Care of Southern Arizona, Surgical Institute of Tucson, Tucson, AZ
Corresponding Author: Morgan Lorio, MD, FACS, Advanced Orthopedics, 499 E. Central Parkway, Altamonte Springs, FL 32701. Phone: (407) 960-1717; Email: mloriomd@gmail.com
Disclosures:
ML: None. OC: None. MR: None. KL: None.
Rationale
The index 2020 ISASS Guideline Statement, Intraosseous Ablation of the Basivertebral Nerve for the Relief of Chronic Low Back Pain (2020), was generated in response to growing requests for background, supporting literature and evidence, as well as proper coding for intraosseous basivertebral nerve ablation (BVNA). Since the guideline was published, the AMA has added CPT® Category I codes for BVNA, 64628 and 64629. Additionally, the CDC has recognized a need for greater specificity in differentiating various types of LBP and has designated ICD-10 CM code M54.51, vertebrogenic low back pain, to ensure correct diagnosis. The timing of these additions provides an opportunity to refresh the ISASS Guideline1 to ensure proper diagnosis and procedural coding, and to update the supporting literature and evidence.
Keywords: Intraosseous ablation, basivertebral nerve, chronic low back pain, vertebrogenic pain
Introduction
Low back pain (LBP) is the most expensive occupational disorder in the United States and according to the 2019 Global Burden of Disease data, LBP is now the leading condition for disability world-wide2. An estimated 49.5 million adults in the United States suffer from chronic LBP with 11% of their life years lived with disability (YLD).3,4 Chronic low back pain (CLBP) is defined as pain that has persisted for greater than 12 weeks. The NIH Pain Consortium adds that CLBP also is present on at least half of the days in the past 6 months.5 Recent longitudinal studies have shown that higher levels of CLBP pain and disability levels were statistically significantly related with poorer health-related quality of life, societal impact, and healthcare costs; with disability from CLBP having a stronger association than pain.6
CLBP direct costs estimates have risen sharply in the past decades from approximately $96 million (for a 12-month period reported in a 2008 claims analysis7) to an estimated $134.5 billion for low back and neck pain spending in 2016; this paid-claims analysis concentrated on adjusted ages from 20 to 64 years, with 57.2% covered by private insurance, 33.7% covered by public insurance, and 9.2% covered by out-of-pocket payments.8 As is the case with many medical conditions, a minority of CLBP patients consume most health care resources. Analyses of commercial payer and Medicare claims databases reveals that 15% of CLBP patients account for 75% of health care costs.9
Clinicians treating axial CLBP have historically been challenged with limited objective differentiators for pain sources, as well as poor effect sizes and a lack of high-quality evidence for existing treatments.10 A lack of validated diagnostic reference standards or specific imaging biomarkers for the various sources of pain leads to a diagnosis of “nonspecific” LBP in 85% of patients. This lack of differentiation in pain sources resulted in large variations in treatment, including overtreatment, with poorly validated, nonspecific therapies (Table 1) and in refractory cases, surgical interventions may be recommended which further drives up the high cost of CLBP treatment.11,12
Table 1– Care management options often used for treating chronic low back pain
- Avoidance of activities that aggravate pain
- Trial of chiropractic manipulation
- Trial of physical therapy
- Cognitive support and recovery reassurance
- Spine biomechanics education
- Specific lumbar exercise program
- Home use of heat/cold modalities
- Low-impact aerobic exercise as tolerated
- Pharmacotherapy (e.g., non-narcotic analgesics, nonsteroidal anti-inflammatory drugs)
- Spinal injections (e.g., epidural steroid injections, medial branch blocks, facet injections) and/or facet ablations
While the disc has been the target for many CLBP treatments, the adjacent vertebral endplates (VEPs) have historically been ignored. A significant body of evidence has accumulated over the past 25 years demonstrating the VEPs are a significant and underappreciated source of CLBP. These studies confirm the presence of pain fibers (nociceptors) in the VEPs that trace back to the basivertebral nerve (BVN) located within the vertebral body and that proliferate with endplate damage.13-20
Damaged VEPs with resulting chronic inflammation are readily visible as Type 1 and/or Type 2 Modic changes (MCs) on routine MRI (Figure 1), a specific biomarker for CLPB. 21-23 Type 1 and Type 2 MCs have been associated with more severe CLBP, higher levels of disability, and worse outcomes from conservative care, leading to higher costs of treatment.24,25
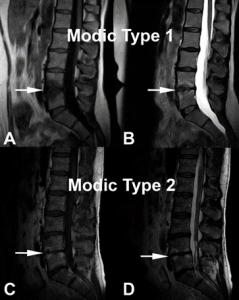
Figure 1. Modic change 1 (MC1) and Modic change 2 (MC2) illustrated at the L4-L5 level.
(A) and (B) demonstrate decreased endplate signal intensity on T1-weighted images and
increased signal intensity on T2-weighted images, respectively (white arrows) corresponding to MC1. (C) and (D) correspond to L4-L5 MC2 characterized by increased endplate signal intensity on T1-weighted images and on T2-weighted images, respectively (white arrows).
Vertebral endplate pain was recently validated by the CDC with ICD-10 code M54-51, Vertebrogenic pain, allowing for a specific diagnosis. It is estimated that 15% of CLBP patients suffer from primary vertebrogenic pain.
Patients with vertebrogenic pain at the L3-S1 levels describe typical anterior column symptoms with midline low lumbar pain, with or without radiation to the paraspinal region and infrequently to the gluteal regions. Pain is exacerbated with sitting, bending (forward flexion), and physical activity.26 This is in contrast to posterior column pain that is associated with primary paraspinal tenderness27 and is exacerbated with spinal extension (e.g., lumbosacral facet joint pain)27 or pain with provocation maneuvers which place sheer, rotational, and/or compressive forces on the sacroiliac joint;28-30 and associated with buttock and posterior thigh pain depending on the age of the patient.28,31
Interruption of pain transmission from the VEPs via destruction of the intraosseous basivertebral nerve using radiofrequency ablation energy (BVN ablation) is a treatment option for patients with vertebrogenic pain. The AMA CPT® Editorial Panel recently recognized the evidence for this minimally invasive outpatient treatment by approving CPT® Category 1 codes for thermal destruction of intraosseous basivertebral nerve, 64628 and 64629.
PUBLISHED LITERATURE
BVN ablation is supported by the following clinical evidence: two Level I clinical trials,32-35 four prospective single arm studies,36-40 a systematic review,41 and a single arm meta-analysis.42
SMART RCT with prospective, single arm 2- and 5-year follow-up
The SMART trial, a prospective randomized, sham-controlled, double-blinded, FDA-Investigational Device Exemption trial was conducted to evaluate the safety and efficacy of BVN ablation for the treatment of CLBP.32,33 A total of 225 CLBP patients with MC1 or MC2 noted in vertebral bodies L3 to S1 were randomized to either a sham control (78 patients) or BVN ablation treatment (147 patients). All study participants were treated with the same operating protocol and pedicle access. The sham-control arm received simulated RF ablation therapy. The primary efficacy endpoint was change in ODI from the baseline to 3 months post-procedure.
Participants in this study were of working age (mean of 47 years), reported severe disability impact from their LBP (mean ODI of 42), and more than 68% had been experiencing CLBP for greater than 5 years. While the intent-to-treat (ITT) population did not achieve significant differences at 3 months due to a high placebo effect with a sham procedure, in the per protocol population (patients with target success and a minimum of follow-up) the mean ODI decreased by 20.5 points for BVN ablation, compared to a 15.2-point decrease in the sham arm (P = 0.019). Response rates for the published MCID of an ODI reduction of 10 or more points were significant between the arms for both the ITT population and the per protocol population in favor of the BVN ablation treatment arm with a 70.1% vs 54.5% (p=0.024) response rate for ITT and 75.6% vs 55.3% response rate (p=0.003) for per protocol. There were no serious device- or procedure-related adverse events reported in patients randomized to the BVN ablation treatment arm through 12 months.
The BVN ablation treatment arm of the SMART trial was followed prospectively at 2- and 5-years post treatment. Of the 128 patients in the per protocol treatment arm of the original SMART trial, 106 (83%) were available for 2-year follow-up.43 Clinical improvements in the ODI, VAS, and the SF-36 physical component summary (PCS) were statistically significant compared with the baseline at all follow-up timepoints through 2 years (3, 6, 9, 12, 18, and 24 months). Patients treated with BVN ablation for CLBP exhibited sustained clinical benefits in ODI and VAS and maintained high responder rates through 2 years following treatment.
The US patients from the original SMART trial were followed for a minimum of 5 years following BVN ablation.44 Of the 117 US treated patients in the original SMART trial, 100 (85%) were available for review with a mean follow-up of 6.4 years (5.4–7.8 years). Mean ODI score improved from 42.81 to 16.86 at 5-year follow-up, a reduction of 25.95 points (p < 0.001). Mean reduction in VAS pain score was 4.38 points (baseline of 6.74, p < 0.001). In total, 66% of patients reported a > 50% reduction in pain, 47% reported a > 75% reduction in pain, and 34% of patients reported complete pain resolution. Composite responder rate for BVN ablation using thresholds of ≥ 15-point ODI and ≥ 2-point VAS for function and pain at 5 years was 75%. At baseline 30/100 patients were actively taking opioids at least once a week; at 5 years, only 8/100 were actively taking opioids for a 73% reduction. At baseline 59/100 patients had received an injection in the prior 12 months to having BVN ablation; at 5 years, 4/100 had received an injection in the prior 12 months and only one had an injection in the region of the BVN ablation.
INTRACEPT RCT with prospective, single arm 1- and 2-year follow-up
The INTRACEPT study 34,35 was a prospective, open label, randomized clinical trial comparing intraosseous BVN ablation to the current standard of care for patients with chronic vertebrogenic low back pain. A total of 140 patients with CLBP of at least 6 months duration with Modic Type 1 or 2 vertebral endplate changes between L3 to S1, were randomized 1:1 using computer generated permuted blocks of six to undergo either BVN ablation or continue standard care. Treatment at up to 4 vertebral bodies was allowed, and 12% of patients had prior discectomy.
At the time of a prespecified interim analysis,34 140 subjects were randomized (66 basivertebral nerve ablation, 74 standard care) at 20 study sites with 104 subjects (n=51 BVN ablation and n=53 standard care) having completed their 3-month primary endpoint visit. Baseline characteristics for all randomized subjects showed a mean age of 49.7 years, a mean ODI of 45.9 (severe impact), a mean VAS of 6.79 (moderate to severe pain), and the percentage of subjects with low back pain symptoms ≥ 5 years of 71.4%. Over 70% of subjects had previously undergone at least one trial of physical therapy or a formal exercise program; 42% had received chiropractic care; and 70% had undergone spinal injections, with 16% having undergone prior RF ablation of a facet or sacroiliac joint(s). Baseline characteristics were similar between the two arms, with no significant differences requiring adjustment in the analysis.
The prespecified interim analysis showed clear statistical superiority (P < 0.001) for all primary and secondary patient-reported outcome measures in the BVN ablation arm compared with the ongoing standard care control arm. 34 This resulted in an independent Data Management Committee recommendation to halt enrollment in the study and offer early crossover to active treatment for the control arm. Comparing the BVN ablation treatment arm with the standard care control arm, the mean changes in ODI at 3 months were −25.3 points versus −4.4 points, respectively, resulting in an adjusted difference of 20.9 points (P <0.001). Changes in mean VAS were −3.46 for BVN ablation versus −1.02 for standard care control, an adjusted difference of 2.44 cm (P <0.001).
The 12-month INTRACEPT publication reported the outcomes of the full randomized cohort of 140 patients.35 The publication included the between arm differences at 3 and 6 months (the point of early cross to active treatment) and the 6-month post treatment outcomes for the control crossover group who demonstrated a nearly identical statistical improvement as the original BVN ablation arm for the 3 and 6-month timepoints. Results from BVN ablation (n=66) remained superior to standard care (n=74), with BVN ablation demonstrating a 25.7-point reduction in mean ODI (p<0.001), and a 3.8 cm VAS reduction (p<0.001) from baseline. Sixty-four percent (64%) of patients receiving BVN ablation treatment reported a ≥50% reduction in VAS and 29% were pain free. Functional outcomes measured via SF-36 and EQ-5D-5L were also significantly reduced at all time points through 12 months from baseline for the BVN ablation arm. Similarly, the former standard care patients who elected to cross to active treatment with BVN ablation (92%) demonstrated a 25.9 point mean ODI reduction (p<0.001) from re-baseline at 6-months post BVN ablation.
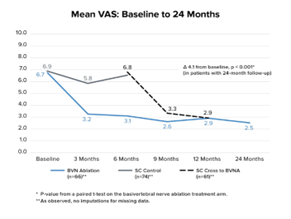
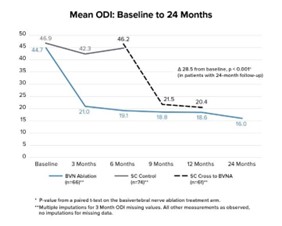
The two-year follow up of the original INTRACEPT trial reported on 58 of the original 66 BVN ablation randomized patients who completed a 24-month visit (88% retention rate).45 Improvements in ODI, VAS, SF-36 PCS, and EQ-5D-5L were statistically significant at all timepoints through 2 years. At 24 months, ODI and VAS improved 28.5 points (from baseline 44.5; p < 0.001) and 4.1 cm (from baseline 6.6; p < 0.001), respectively. A combined responder rate of ODI ≥ 15 and VAS ≥ 2 was 73.7%. A ≥ 50% reduction in pain was reported in 72.4% of treatment arm patients and 31.0% were pain-free at 2 years. At 24 months, only 3 (5%) of patients had steroid injections at the same level as the BVN ablation, and 62% fewer patients were actively taking opioids. There were no serious device or device-procedure related adverse events for BVN ablation reported through 24 months of follow-up. See Figure 2 for the mean ODI and VAS for each study time point through 24 months of follow-up.
Figure 2 – Mean ODI and Mean VAS Over Time – These graphs depict the mean ODI and mean VAS at each study follow-up for each arm. A statistically significant and clinically meaningful difference between arms in mean ODI and VAS improvement was demonstrated at 6 months as well as from baseline/re-baseline for each timepoint in patients treated with BVN ablation, including in control patients that crossed to active treatment.
Multi-center, Prospective, Single Arm Cohort Study
A single arm prospective, single-arm, multicenter, open-label cohort study to evaluate the effectiveness of intraosseous BVN ablation for the treatment of primary vertebrogenic-related CLBP (identified by clinical assessment and Type 1 or Type 2 Modic Changes at L3 to S1) was conducted in two typical spine practice settings with more permissive inclusion of typical CLBP patients (such as patients who have had prior discectomy and users of extended-release opioids).37,38 The primary endpoint was patient-reported change in ODI from the baseline to 3 months post procedure. Secondary outcome measures included change in LBP pain VAS, Short Form 36 (SF-36), EQ-5D-5L, and response rates.
An interim analysis was conducted with approximately 60% of the treated patients completed their 3-month primary endpoint visit.37 The median age of the n=28 interim analysis population was 45 years and baseline values for ODI and VAS 48.5 and 6.36 cm (on a 0 to 10 cm scale) respectively demonstrating a severe level of disability and pain within this interim analysis population. Seventy-five percent of the study patients reported LBP symptoms for ≥5 years with 25% actively using opioids and 61% previously treated with injections. Clinically meaningful and statistically significant improvements were demonstrated in all outcome measures at the 3-month primary endpoint. Mean reduction in ODI from the baseline at 3 months post treatment was -30.07 ± 14.52 points (P < .0001). The mean reduction in VAS pain score from the baseline was -3.50 ± 2.33 (P < .0001). Using a minimal clinically important difference (MCID) of ≥10-point improvement in ODI, 93% of patients were responders; using MCID of a ≥20-point improvement in ODI, 75% were responders. Likewise, VAS MCID of a ≥2.0 cm reduction was achieved in 75% of patients. Importantly, in this population of working-aged individuals, 83% reported improvement in work function.
A one-year follow up of the full cohort for this single arm prospective study included 45 of 47 patients (retention rate of 96%) has been published.38 Patients demonstrated a mean reduction in ODI of 32.31 (P<0.001) with 88.89% (40/45) patients reporting a ≥15-point ODI decrease at 12 months. Mean VAS pain score decreased 4.31 at 12 months (P<0.001) and more than 69% reporting a 50% reduction in VAS pain scale. Similarly, SF-36 and EQ-SD-5L scores improved 26.27 and 0.22, respectively (each P<0.001).
Pain and functional improvements post BVN ablation have been demonstrated as both reproducible and durable in the above two RCTs and are further confirmed in the prospective single arm cohort study of approximately 50 typical spine patients from two community spine practices. See Figures 3 and 4.
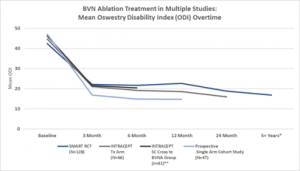
Figure 3 Multi-Study Comparison of Functional Improvement – Comparison of mean ODI over time for the two Level I RCTs and the CLBP single arm study.32, 34-35, 37
*SMART RCT US per protocol treatment arm at mean of 6.4 years
**Standard arm re-baselined and offered active treatment at a median of 5.8 months
Figure 4 – Comparison of proportion of patients by percent reduction in VAS for the two Level I RCTs and the CLBP single arm study.32,35,37
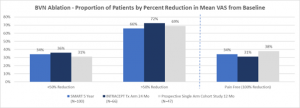
DeVivo et al – Prospective, Single Arm Study (2020)
This independent study assessed the feasibility and safety of percutaneous CT-guided basivertebral nerve ablation.39 Basivertebral nerve ablation was performed in 56 consecutive patients presenting with vertebrogenic chronic LBP using an articulating bipolar radiofrequency electrode (STAR Tumor Ablation System Merit) off-label. Patients were evaluated at 3- and 12-months post-treatment using a composite endpoint of clinical success, defined as an improvement in VAS ≥2 cm and an improvement in ODI ≥10 points.
The CT-assisted technique was determined to be successful in all patients after assessment of the ablation zone. At 3- and 12-month follow-up, VAS and ODI scores decreased significantly compared to baseline. Clinical success was reached in 54/56 patients (96.5%) for pain and 54/56 patients (96.5%) for disability. Mean VAS decreased 4.3 cm (range 1 to 7.5), with ODI decreasing 32.4 (range 6 to 42) points.
Fishchenko et al – Single Group Observational Study (2021)
This independent prospective study evaluated the outcomes of 19 patients selected for BVNA based upon > 6 months of low back pain, Modic I and II from L3-S1, no improvement following drug treatment, and an ODI > 30 and VAS > 4.40 These subjects were followed for a minimum of 12 months. After 12 months, patients were observed to have a mean decrease in VAS of 5.2 cm from a baseline of 7.6 cm (68% reduction), and a mean improvement in ODI of 27.5 points from a baseline of 49.2 (56% reduction).
Conger et al – Systematic Review (2021)
A systematic review of BVNA was published in 2021.41 Of the 725 publications screened for the analysis, seven publications with 321 participants were ultimately included. The reported 3-month success rate for ≥50% pain reduction ranged from 45% to 63%. Rates of functional improvement (≥10-point ODI improvement threshold) ranged from 75% to 93%. For comparison to sham treatment, the relative risk of treatment success defined by ≥50% pain reduction and ≥10-point ODI improvement was 1.25 (95% confidence interval [CI]: .88-1.77) and 1.38 (95% CI: 1.10-1.73), respectively. For comparison to continued standard care treatment the relative risk of treatment success defined by ≥50% pain reduction and ≥10-point ODI improvement was 4.16 (95% CI: 2.12-8.14) and 2.32 (95% CI: 1.52-3.55), respectively.
The systematic review concluded there is moderate-quality evidence that suggests this procedure is effective in reducing pain and disability in patients with chronic low back pain who are selected based on type 1 or 2 Modic changes, among other inclusion and exclusion criteria used in the published literature to date. Success of the procedure appears to be dependent on effective targeting of the BVN.
Conger et al have recently had an updated systematic review of the literature with a single arm meta-analysis accepted for publication.42 Of the 856 unique records screened, 12 publications met inclusion criteria, representing six unique study populations, with 414 participants allocated to receive BVN ablation. Single-arm meta-analysis showed a success rate of 65% (95% CI 51-78%) and 64% (95% CI 43-82%), for ≥50% pain relief at six and 12-months, respectively. Rates of ≥15-point ODI improvement were 75% (95%CI 63-86%) and 75% (95%CI 63-85%) at six and 12 months, respectively.
Procedure Safety
In the studies originating from the United States and listed with clinicaltrials.gov, safety data has been collected in 473 clinical trial patients. There has been 1 serious device procedure-related event reported (0.2%); a vertebral compression fracture in a sham-control crossover patient with osteopenia, taking hormone therapy. The fracture healed spontaneously by 8 weeks. There have been 26 non-serious device-procedure related events reports (5.5%). The most common was increase in back pain, onset of leg pain (radiculitis/radiculopathy). All non-serious events were transient in nature with a median time to resolution of 66.5 days and were typically treated with oral medication.41,42,46,47
In review of serious adverse events in the MAUDE database, one incidence of procedure-related retroperitoneal hematoma and one incidence of post-procedure endplate fracture were reported since commercialization of the device in 2018.48
Evidence and Literature Conclusion
Intraosseous ablation of the BVN is supported by basic and clinical evidence foundation, including a systematic review; a level 1, sham controlled RCT, a second level 1 RCT against standard conservative management, three single group prospective studies and a post-hoc secondary analysis. Outcomes data greater 5 years (mean 6.4 years) following a single BVN ablation procedure suggest durability of the treatment effect.
Evidence from the Level I studies also indicate that BVN ablation may assist in decreasing the need for opioids to manage axial low back pain. Additionally, it appears that successful BVN ablation decreases the need for additional treatment (spinal injections and future invasive surgery) in the region where BVN ablation was performed. (Please refer to table 2 at the end of this review).
Collectively, the studies reviewed demonstrate that BVN ablation provides clinically meaningful improvements in pain and function at 5+ years with an excellent safety profile. This evidence supports BVN ablation as a treatment option for a well-defined subpopulation of CLBP patients.
INDICATIONS/LIMITATIONS OF COVERAGE
Intraosseous ablation of the BVN from the L3 through S1 vertebrae may be considered medically indicated for individuals with CLBP when all the following criteria are met:
- CLBP of at least 6 months duration,
- Failure to respond to at least 6 months of nonsurgical management, and
- MRI-demonstrated MC1 or MC2 in at least 1 vertebral endplate at 1 or more levels from L3 to S1.
BVNA is NOT indicated in the following:
- Patients with severe cardiac or pulmonary compromise.
- Presence of implanted pulse generator(s) (e. g., pacemaker, defibrillator)/electronic implants except for circumstances where a specific patient safety precaution may be implemented.
- Co-existence of other obvious radiographic etiology for patient’s axial CLBP requiring a medically necessary surgical intervention or precluding
- Active or chronic infection–systemic or local.
- Patients who are pregnant.
- Skeletally immature patients (generally < 18 years of age).
- Current or post-trauma, tumor, infection, or poor bone quality compromising vertebral pedicle/body.
- Cauda equina syndrome defined as neural compression causing neurogenic bowel or bladder dysfunction.
- Radiographic confirmation of gross spinal instability including angular or translatory instability (grade 2 or greater spondylolisthesis) at index level(s).
- Morbid obesity precluding satisfactory procedural imaging.
- Targeted ablation zone is < 10mm away from a sensitive structure not intended for ablation or implanted hardware precludes clinical access or is within the ablation zone.
- Situation where unintended tissue damage may result based on the clinical assessment by the physician.
- Application with electrosurgical instruments NOT tested and specified for use with the current FDA clearance for the Relievant RFG.
CODING AND COVERAGE HISTORY
Intraosseous ablation of the BVN is a procedure commercially performed since 2018. Since January 1, 2022, the procedure is reported with American Medical Association Current Procedural Terminology (CPT®) codes 64628 and 64629.
64628: Thermal destruction of intraosseous basivertebral nerve, including all imaging guidance; first 2 vertebral bodies, lumbar or sacral
64629: each additional vertebral body, lumbar or sacral (list separately in addition to code for primary procedure.
Patients indicated for the procedure may be described diagnostically by International Classification of Diseases, 10th Revision codes for medical necessity are as follows:
M47.816: Spondylosis without myelopathy or radiculopathy, lumbar region,
M47.817: Spondylosis without myelopathy or radiculopathy, lumbosacral region,
M51.36: Other intervertebral DD, lumbar,
M51.37: Other intervertebral DD, lumbosacral,
M54.50: Low back pain
M54.51: Vertebrogenic low back pain
PHYSICIAN QUALIFICATIONS
- Intraosseous basivertebral nerve ablation is a surgical procedure that may be performed by physicians with spinal expertise and advanced training in pedicular access.
- Such spinal specialists have successfully completed a residency/fellowship in their specialty and have participated in a specialized training course under the supervision of a physician experienced in the procedure using specimens that permit hands-on experience with the surgical technique.
- At this time, the procedure should be performed in either the hospital outpatient setting (HOPD) or ambulatory surgical center (ASC) where either general anesthesia or moderate conscious sedation (MAC) is available.
COVERAGE/CONCLUSION
The utilization of intraosseous basivertebral nerve ablation to address vertebrogenic low back pain has become a recognized safe, predictable, and durable surgical method for the management of chronic axial low back pain identified using well-established clinical and MRI findings, Modic type 1 and/or type 2 changes. The procedure is supported by Level I evidence including a systematic review and 2 RCTs demonstrating a statistically significant decrease in pain and an improvement in function with outcomes sustained greater than 5 years after a single treatment. These results were seen in a patient population that is one of the most expensive and difficult to provide care for. In this era of rising healthcare costs and increasing need for therapies to reduce the use of opioids, BVN ablation may provide a treatment option to fill the treatment gap paradigm for patients that fail non-surgical treatment.
The ISASS policy does not endorse any specific system to perform the procedure and has made its recommendation that vertebrogenic low back pain is most successfully addressed by intraosseous ablation of the basivertebral nerve. This was based upon the analysis of peer-reviewed publications (see below), including two international studies (DeVivo and Fishchenko). However, at this time there is only one FDA 510(k) system cleared for performing intraosseous basivertebral nerve ablation in the United States.
Table 2– Supporting Literature & Evidence
Author, year Design Study Size Inclusion Criteria Mean Age of Participants (range) Participant duration of Pain Targeting Success (based upon post BVNA MRI) Adverse Events Becker 2017 SGOS 16 CLBP >6 months Modic 1 or 2 changes L3 – S1 or positive discography Mean 48.0 (34 – 66)
Not reported 91% n=4, lumbar pain, buttock pain, dysesthesia, and transient numbness,
resolved with pain medications.
Fischgrund 2018 RCT 225 randomized, 147 received BVN Ablation, 128 PP (87%) at 12 months of follow-up
CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 46.9 (26-69) 6-12 months – 4%, 1-2 years -10%,
2-3 years – 7%,
3-5 years -12%,
> 5 years – 67%
95% n= 1 nerve root injury (sham group), n=1 vertebral compression fracture (sham
group), n=1 retroperitoneal hemorrhage (sham group), n=7 lumbar radiculitis,
and transient motor or sensory deficits, all resolved with supportive care.
Fischgrund 2019 SGOS 106 of 128 PP BVN Ablation (83%) at 24 months of follow-up CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 47.4 (27-69) 6-12 months – 5%, 1-2 years -11%,
2-3 years – 6%,
3-5 years -14%,
> 5 years – 64%
89% Previously discussed. No additional serious or related adverse events reported through 24 months of follow-up. Fischgrund 2020 SGOS 100 of 117 PP BVN Ablation US population (85%) at 5+years of follow-up CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 47.2 (26 – 69) 6–12 months – 4%, 1–2 years – 11%,
2–3 years – 4%,
3–5 years – 12%,
>5 years – 69%
89% Previously discussed. No additional serious or related adverse events reported through a mean of 6.4 years of follow-up. Khalil 2019 RCT 140 total randomized 51 of 66 randomized to BVN Ablation treatment arm with a 3 month primary endpoint visit completed (Interim Analysis Population)
CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 50.0 (26 – 70)
6-12 months – 8%, 1–2 years – 6%,
2–3 years – 10%,
3–5 years – 14%,
>5 years – 63%
96% Interim Analysis reported events: n=15, incisional pain, leg pain/paresthesia, back pain in a new
location, urinary retention, and lateral
femoral cutaneous neurapraxia. All resolved.
Smuck 2021 RCT All BVN Ablation Treated (at 12 months): 61 of 66 BVN Ablation treatment arm
at 12 months of follow-up (92%)
61 of 74 standard care controls that crossed to active treatment (82% crossover rate)
CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 BVN Ablation Mean 49.4 (30-68) Crossover Mean 49.5
(26-70)
6–12 months – 6%, 1–2 years – 6%,
2–3 years – 9%,
3–5 years – 15%,
>5 years – 64%
6–12 months – 3%,
1–2 years – 0%,
2–3 years – 10%,
3–5 years – 7%,
>5 years – 80%
97% Full cohort events through 12 months of follow-up: n=21, 1 incisional pain, 1 nausea and 1 inability to complete the procedure related to anesthesia, 1 urinary retention, 1 incision infection, 4 back pain related to procedure positioning, 13 leg pain/paresthesia (resolved median 43 days with oral medication).
Koreckij 2021 SGOS 58 of 66 BVN Ablation treatment arm at 24 months of follow-up (88%)
CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 50.4 (30-68) 6–12 months – 3%, 1–2 years – 5%,
2–3 years – 9%,
3–5 years – 16%,
>5 years – 67%
98% Previously discussed. No additional serious or related events through 24 months of follow-up. Truumees 2019 SGOS 28 of 48 BVN Ablation single arm with 3 month primary endpoint visit (Interim Analysis Population) CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 45.2 (SD 8.89) 6–12 months – 0%, 1–2 years – 11%,
2–3 years – 14%,
3–5 years – 0%,
>5 years – 75%
97% n=3, 1 aborted procedure due to inability to access, 2 leg pain events due to pedicle breach, resolved with oral medication Macadaeg 2020 SGOS 45 of 48 BVN Ablation (Full Cohort) with 12 month visit (94%) CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Median 45.0 (25 – 66) 1 – 2 years 14.9%, 2 – 3 years 10.6%, 3 – 5 years 2.1%, > 5 years 72.3% 96% Full cohort through 12 months of follow-up adverse events: n=5, 1 aborted procedure due to inability to access, 3 radiculitis associated with potential pedicle breach resolved with oral medications, 1 corneal abrasion, 1 skin reaction to surgical prep
DeVivo 2020 SGOS 56 CLBP > 6 months despite > 6 weeks treatment, with Modic 1 or 2 changes L3 – S1 Median 43.0 (38-52)
Not reported 100% None Fishchenko 2021 SGOS 19 CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 52.6 (SD 6.9) 1 – 2 years 73.7%, > 5 years 26.3% Not reported n=1, arterial injury of the “lumbalis sinistra” causing a hematoma within the iliopsoas with associated plexitis, treated with endovascular embolization
Markman 2019 PSA 225 randomized, 147 received BVNA, 128 PP CLBP >6 months despite treatment with Modic 1 or 2 changes L3 – S1, minimum ODI 30, VAS 4 Mean 46.9 (26-69) 6-12 months – 4%, 1-2 years -10%,
2-3 years – 7%,
3-5 years -12%,
> 5 years – 67%
95% Not reported References
- Lorio M, Clerk-Lamalice O, Beall DP, Julien T. International Society for the Advancement of Spine Surgery Guideline-Intraosseous Ablation of the Basivertebral Nerve for the Relief of Chronic Low Back Pain. Int J Spine Surg. 2020 Feb 29;14(1):18-25. doi: 10.14444/7002. PMID: 32128298; PMCID: PMC7043835.
- Institute for Health Metrics and Evaluation (IHME). United States Profile. Seattle, WA: IHME, University of Washington, 2018. Available from http://www.healthdata.org/node/5300. Accessed August 18, 2021.
- Wu A, March L, Zheng X, Huang J, Wang X, Zhao J, Blyth FM, Smith E, Buchbinder R, Hoy D. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020 Mar;8(6):299. doi: 10.21037/atm.2020.02.175. PMID: 32355743; PMCID: PMC7186678
- Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet. 2018 Jun 9;391(10137):2356-2367. doi: 10.1016/S0140-6736(18)30480-X. Epub 2018 Mar 21. PMID: 29573870.
- Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH Task Force on research standards for chronic low back pain. J Pain. 2014;15(6):569-585. doi:10.1016/j.jpain.2014.03.005
- Mutubuki EN, Beljon Y, Maas ET, Huygen FJPM, Ostelo RWJG, van Tulder MW, et al. The longitudinal relationships between pain severity and disability versus health-related quality of life and costs among chronic low back pain patients. Qual Life Res. 2020;29(1):275-287. doi:10.1007/s11136-019-02302-w
- Maneesha Mehra, Kala Hill, Deborah Nicholl & Jan Schadrack (2012) The burden of chronic low back pain with and without a neuropathic component: a healthcare resource use and cost analysis, Journal of Medical Economics, 15:2, 245-252, DOI: 10.3111/13696998.2011.642090
- Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al.US Health Care Spending by Payer and Health Condition, 1996–2016. 3 March 2020. doi:10.1001/jama.2020.0734.
- MarketScan, Truven Health Analytics from October 2011 to September 2016.
- Keller A, Hayden J, Bombardier C, van Tulder M. Effect sizes of non-surgical treatments of non-specific low-back pain. Eur Spine J. 2007;16(11):1776-1788. doi:10.1007/s00586-007-0379-x
- Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-8. DOI:10.3122/jabfm.2009.01.080102
- Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173(17):1573-81. DOI: 10.1001/jamainternmed.2013.8992.
- Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153.
- Antonacci MD, Mody DR, Heggeness MH. Innervation of the human vertebral body: a histologic study. J Spinal Disord. 1998;11:526–531.
- Fagan A, Moore R, Vernon-Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: The innervation of the intervertebral disc: a quantitative analysis. Spine 2003;28:2570-2576. doi:10.1097/01.BRS.0000096942.29660.B1
- Fras C, Kravetz P, Mody DR, Heggeness MH. Substance P-containing nerves within the human vertebral body. an immunohistochemical study of the basivertebral nerve. Spine J. 2003;3(1):63-67. doi: 10.1016/s1529-9430(02)00455-2.
- Bailey JF, Liebenberg E, Degmetich S, et al. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat 2011;218(3):263-70. DOI:10.1111/j.1469-7580.2010.01332.x
- Lotz JC, Fields AJ, Liebenberg EC. The Role of the Vertebral End Plate in Low Back Pain. Global Spine J 2013;03(3):153-64. DOI: 10.1055/s-0033-1347298
- Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral endplate and intervertebral disc. Spine J. 2014 March 1; 14(3): 513–521. DOI:10.1016/j.spinee.2013.06.075.
- Degmetich S, Bailey JF, Liebenberg E, Lotz JC. Neural innervation patterns in the sacral vertebral body. Eur Spine J. 2016;25(6):1932-1938. doi:10.1007/s00586-015-4037-4.
- Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi: 10. 1148/radiology.166.1.3336678.
- Dudli S, Fields AJ, Samartzis D, et al. Pathobiology of Modic changes. Eur Spine J 2016;25(11):3723-34. DOI: 10.1007/s00586-016-4459-7
- Dudli S, Sing DC, Hu SS, et al. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J 2017;26(5):1362-73. DOI: 10.1007/s00586-017-4955-4.
- Kjaer P, Korsholm L, Bendix T, et al. Modic changes and their associations with clinical findings. Eur Spine J 2006;15(9):1312-9. DOI: 10.1007/s00586-006-0185-x
- Bailey JF, Fields AJ, Ballatori A, Cohen D, Jain D, Coughlin D, O’Neill C, McCormick Z, Han M, Krug R, Demir-Deviren S, Lotz JC. The Relationship Between Endplate Pathology and Patient-reported Symptoms for Chronic Low Back Pain Depends on Lumbar Paraspinal Muscle Quality. Spine (Phila Pa 1976). 2019 Jul 15;44(14):1010-1017.
- McCormick ZL, Sperry BA, Lotz JC, Boody BS, Harper K, Burnham T. Pain Location and Exacerbating Activities Associated with Treatment Success Following Basivertebral Nerve Ablation: An Aggregated Cohort Study of Multicenter Prospective Clinical Trial Data. Accepted for Publication Pain Med. 2022.
- Cohen SP, Bhaskar A, Bhatia A, Buvanendran A, Deer T, Garg S, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med. 2020 Jun;45(6):424–67.
- DePalma MJ, Ketchum JM, Saullo TR. Multivariable analyses of the relationships between age, gender, and body mass index and the source of chronic low back pain. Pain Med. 2012 Apr 1;13(4):498–506.
- Depalma MJ, Ketchum JM, Trussell BS, Saullo TR, Slipman CW. Does the location of low back pain predict its source? PMR. 2011;3(1):33–9.
- Hancock MJ, Maher CG, Latimer J, Spindler MF, McAuley JH, Laslett M, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007 Oct 15;16(10):1539–50.
- DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011 Feb 1;12(2):224–33.
- Fischgrund JS, Rhyne A, Franke J, Sasso R, Kitchel S, Bae H, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2018;27(5):1146-56. DOI: 10.1007/s00586-018-5496-1.
- Markman JD, Rhyne AL, Sasso RC, Patel AA, Hsu WK, Fischgrund JS, et al. Association between opioid use and patient-reported outcomes in a randomized trial evaluating basivertebral nerve ablation for the relief of chronic low back pain. Neurosurgery. 2019 Apr 29;86(3):343–7.
- Khalil J, Smuck M, Koreckij T, Keel J, Beall D, Goodman B, et al. A prospective, randomized, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019 Jun 20. pii: S1529-9430(19)30800-9. doi: 10.1016/j.spinee.2019.05.598
- Smuck M, Khalil JG, Barrett K, Hirsch JA, Kreiner S, Koreckij T, et al. A prospective, randomized, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. Reg Anesth Pain Med. 2021;rapm-2020-102259. doi:10.1136/rapm-2020-102259.
- Becker S, Hadjipavlou A, Heggeness MH. Ablation of the basivertebral nerve for treatment of back pain: a clinical study. Spine J 2017;17(2):218-23. DOI: 10.1016/j.spinee.2016.08.032
- Truumees E, Macadaeg K, Pena E, Arbuckle A, Gentile, J, Funk R, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. European Spine Journal 2019, https://doi.org/10.1007/s00586-019-05995-2.
- Macadaeg K, Truumees E, Boody B, Pena E, Arbuckle A., Gentile, J, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. NASSJ 2020;3(100030). E-pub 18 Sept 2020. https://doi.org/10.1016/j.xnsj.2020.100030
- DeVivo AE, D’Agostino G, D’Anna Al Qatami H, Gil I, Ventura F, Manfre L, Intraosseous basivertebral nerve radiofrequency ablation (BVA) for treatment of vertebrogenic chronic low back pain Neuroradiology 2021 May; 63(5): 809-815. DOI: 1007/s00234-020-02577-8
- Fishchenko I.V., Garmish A.R., Kravchuk L.D., Saponenko A.I. Radiofrequency ablation of the basivertebral nerve in the treatment of chronic low back pain: analysis of a small clinical series. Hirurgiâ pozvonočnika (Spine Surgery). 2021;18(3):61-67. https://doi.org/10.14531/ss2021.3.61-67
- Conger A, Schuster NM, Cheng DS, Sperry BP, Joshi AB, Haring RS, Duszynski B, McCormick ZL. The effectiveness of intraosseous basivertebral nerve radiofrequency neurotomy for the treatment of chronic low back pain in patients with Modic changes: a systematic review. Pain Med. 2021 May 21;22(5):1039-1054. doi: 10.1093/pm/pnab040. PMID: 33544851.
- Conger A, Burnham T, Clark T, McCormick Z. The effectiveness of intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebrogenic low back pain: an updated systematic review with single arm meta-analysis. Pain Med accepted for publication 2022.
- Fischgrund JS, Rhyne A, Franke J, Sasso R, Kitchel S, Bae H, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: two-year results from a prospective randomized double-blind sham- controlled multi-center Study. Int J Spine Surg 2019;13(2).
- Fischgrund JS, Rhyne A, Macadaeg K, Moore G, Kamrava E, Yeung C, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2020 Aug;29(8):1925-1934. DOI: 10.1007/s00586-020-06448-x.
- Koreckij T, Kreiner S, Khalil JG, Smuck M, Markman J, Garfin S. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-month treatment arm results. NASSJ. Published online October 26, 2021. DOI: https://doi.org/10.1016/j.xnsj.2021.100089
- gov https://clinicaltrials.gov/ct2/home. Accessed March 30, 2022.
- Clinical studies aggregate device and procedure-related adverse event data supplied by Relievant Medsystems, Inc. March 2022 for studies.
- Medical Device Reports for FDA MAUDE online database. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed on March 30, 2022.
Disclosures: None
